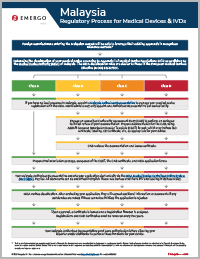
Malaysia Regulatory Approval Process for Medical Devices
有任何问题?向我们的专家获取相关信息
联系我们The chart shown illustrates Malaysia's approval process and is available for download in PDF format. However, if you would like to explain the steps to someone else in an email, you can cut and paste the text below or send them a link to this page.
Malaysia's medical device approval process explained
Foreign manufacturers entering the Malaysian market will be able to leverage their existing approvals in recognized reference markets (Australia, Canada, European Union countries, Japan, and the United States).*
Step 1
Determine the classification of your medical device according to Appendix I of Medical Device Regulations 2012 as published by the Medical Device Authority (MDA) of Malaysia. The MDA classification rules are similar to those in the European Medical Devices Directive (MDD) 93/42/EEC.
Step 2
If you have no local presence in Malaysia, appoint a Malaysia Authorized Representative to manage your medical device registration with the MDA. Manufactures may only appoint one Authorized Representative per device family.
Step 3
For classes B, C, and D, engage an accredited Conformity Assessment Body (CAB) to perform an abridged technical review of your documentation. Prepare documentation for the CAB using ASEAN Common Submission Dossier Template (CSDT) format, which may include ISO certificate, labeling, CE Certificate, etc., as appropriate for your device.
Step 4
CAB reviews the documentation and issues certificate.
Step 5
For all devices, prepare final submission package, composed of the CSDT, the CAB certificate, and MDA application forms.
Step 6
Your Malaysia Authorized Representative submits your application electronically via the MDA Medical Device Centralized Online System (MeDC@St). Pay fee. All documents can be submitted in English. Devices for home use must have IFU and labeling in Bahasa Malay.
Step 7
MDA verifies classification. After evaluating your application, they will request additional information or documents if any deficiencies are noted; if these cannot be fulfilled, the application is rejected.
Step 8
Upon approval, a Certificate is issued and a Registration Number is assigned. Registrations and CAB Certificates must be renewed every five years.
Step 9
Your Malaysia Authorized Representative must issue authorization letters allowing your importer and/or distributor to perform those functions for your device.
This is a simplified overview of the process.
* This chart demonstrates the route to compliance in Malaysia for devices that have already obtained approval in a reference market. Recognized reference markets include: Australia, Canada, European Union countries, Japan, and the United States. This is not a requirement and an approval process does exist for imported devices with no reference market approval; however, the process in Malaysia will be eased by already having such approvals.
Chart updated: 06/24/2019
相关
-
美国FDA eSTAR电子递交模板与资源要求解析
自2018年起,美国食品和药物管理局(U.S. Food and Drug Administration,FDA)开始试行以电子化方式来帮助行业提供完整的510(k)上市前通知。2022年,为推进以电子格式提供510(k)递交文件的过渡,美国FDA正式发布关于使用电子递交模板和资源的最终指导文
阅读更多 -
针对体外诊断医疗器械的欧盟IVDR符合性评定选项
自2022年5月26日始,欲在欧盟(EU)上市的新型体外诊断(IVD)医疗器械必须符合欧盟体外诊断医疗器械法规(2017/746 IVDR)。 同日起,带有有效CE标志的IVD可以继续按照指令98/79/EC(IVDD)进行销售,直至其许可证到期为止。 自2025年5月27日始,在欧盟销售的所
阅读更多